T cell activation is a crucial process in the immune system, enabling the body to fight infections and diseases. T cells, a type of white blood cell, are activated when their receptors recognize specific antigens presented by antigen-presenting cells (APCs) such as dendritic cells. This recognition occurs through the binding of the T cell receptor (TCR) to the antigen-MHC complex on the APC.
Upon activation, T cells undergo clonal expansion, producing millions of cells that target the specific antigen. This process involves two key signals: the first is the TCR binding to the antigen-MHC complex, and the second is the co-stimulatory signal provided by molecules like CD28 binding to B7 on the APC. Activated T cells then differentiate into various subtypes, including helper T cells (CD4+) and cytotoxic T cells (CD8+), each playing distinct roles in the immune response.This activation is essential for an effective immune response, ensuring that the body can identify and eliminate pathogens efficiently.
What is T Cell
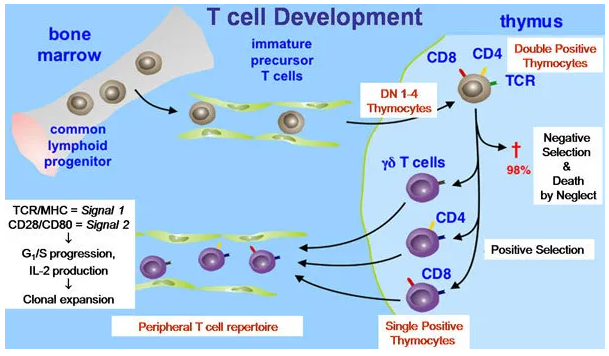
T cells, or T lymphocytes, are a type of white blood cell essential for the immune system. Originating in the bone marrow, they mature in the thymus, hence the name "T cells". These cells play a central role in the adaptive immune response, which is the body’s way of targeting specific pathogens.
There are several types of T cells, each with distinct functionsHelper T cells (CD4+) assist other immune cells by releasing cytokines, which enhance the immune response Cytotoxic T cells (CD8+) directly kill infected or cancerous cells Regulatory T cells help maintain immune tolerance, preventing autoimmune reactions.
T cells recognize antigens through their T cell receptors (TCRs), which bind to fragments of pathogens presented by other cells. This recognition triggers T cell activation, leading to the proliferation and differentiation of T cells to effectively combat the infection.
The main types of T cells and their functions
T cells, a type of white blood cell, are vital for the immune system. There are three main types: Helper T cells (CD4+), Cytotoxic T cells (CD8+), and Regulatory T cells.
Helper T cells (CD4+):play a crucial role by releasing cytokines that signal other immune cells, such as B cells and macrophages, to enhance the immune response. They are essential for coordinating the body’s defense against pathogens.
Cytotoxic T cells (CD8+):directly attack and destroy infected or cancerous cells by inducing apoptosis, a process of programmed cell death. This makes them key players in eliminating cells that harbor intracellular pathogens like viruses.
Regulatory T cells:help maintain immune tolerance by suppressing the activity of other T cells, preventing autoimmune reactions where the immune system might attack the body’s own tissues.
These T cell types collectively ensure a balanced and effective immune response, protecting the body from infections and diseases.
What is T cell activation?
T cell activation is a crucial process in the adaptive immune response, enabling the body to recognize and combat pathogens. T cells, a type of white blood cell, originate from hematopoietic stem cells in the bone marrow and mature in the thymus. They play a central role in identifying and eliminating infected or cancerous cells.
The Activation Process
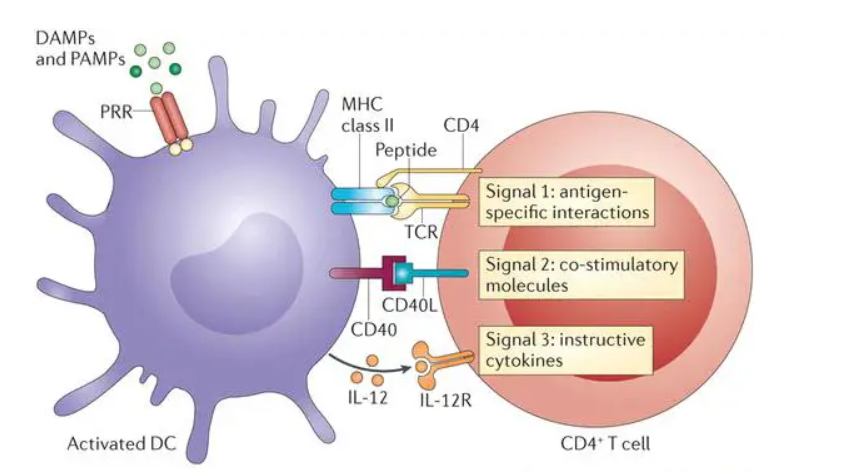
T cell activation involves a series of steps initiated when a T cell receptor (TCR) recognizes a specific antigen presented by an antigen-presenting cell (APC). This interaction occurs within the major histocompatibility complex (MHC) on the APC’s surface. The process can be divided into three main signals:
Signal One: The TCR binds to the antigen-MHC complex, providing the initial activation signal. This binding is stabilized by CD4 or CD8 molecules, depending on whether the T cell is a helper (CD4+) or cytotoxic (CD8+) T cell.
Signal Two: Co-stimulatory molecules on the T cell, such as CD28, bind to their counterparts (e.g., CD80 or CD86) on the APC. This signal is essential for T cell proliferation and differentiation. Without this co-stimulation, T cells may become anergic or unresponsive.
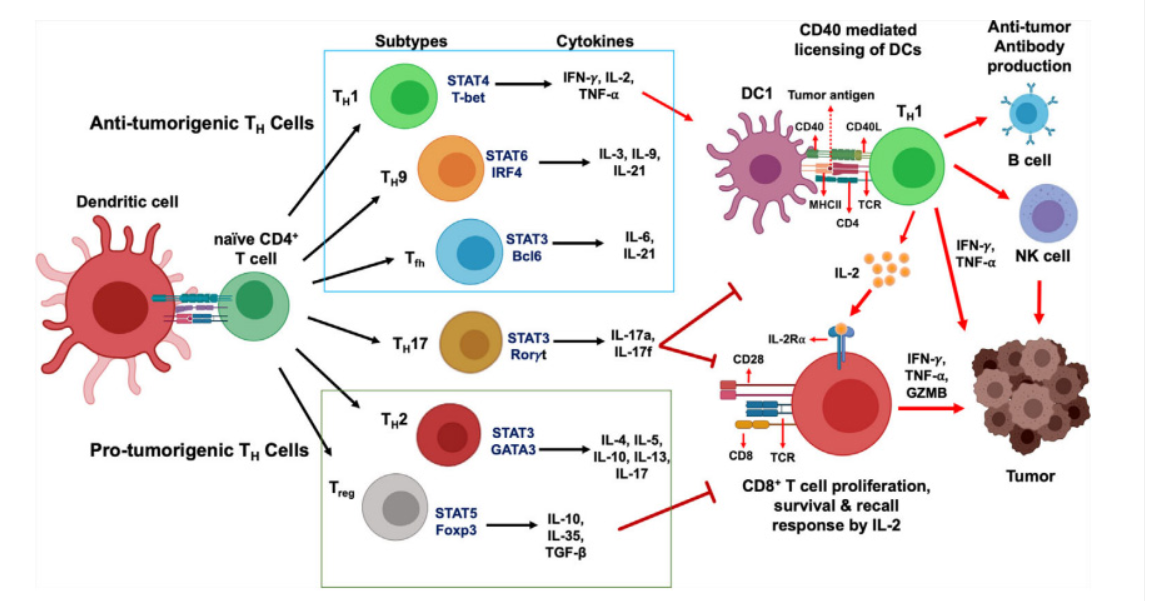
Signal Three: Cytokines released by the APC and other immune cells further direct the T cell’s response. These cytokines influence the differentiation of T cells into various subtypes, such as Th1, Th2, Th17, or regulatory T cells, each with distinct functions in the immune response.
Importance and Implications
Activated T cells proliferate and differentiate into effector cells that can directly kill infected cells (cytotoxic T cells) or help other immune cells (helper T cells). This activation is tightly regulated to prevent inappropriate immune responses, which could lead to autoimmunity.Understanding T cell activation is vital for developing immunotherapies and vaccines. For instance, enhancing T cell responses can improve cancer immunotherapy, while modulating T cell activity can help treat autoimmune diseases.
T cell activation is a complex but well-orchestrated process essential for effective immune defense, involving precise interactions and signals that ensure a targeted and regulated immune response.
T cell activation markers
T cell activation markers are crucial indicators used to identify and study the activation status of T cells, a type of white blood cell essential for the immune response. Upon encountering an antigen, T cells undergo a series of changes, including the expression of specific surface proteins known as activation markers.
Key Activation Markers:
CD69: One of the earliest markers to appear on the surface of activated T cells. It is transiently expressed and plays a role in the retention of T cells in lymphoid tissues.
CD25: Also known as the interleukin-2 receptor alpha chain, CD25 is upregulated following T cell activation and is involved in the proliferation and survival of T cells.
CD71: The transferrin receptor, CD71, is essential for iron uptake and is upregulated during T cell activation, reflecting increased metabolic activity.
CD40L (CD154): Expressed on activated CD4+ T cells, CD40L interacts with CD40 on antigen-presenting cells, enhancing the immune response.
These markers are used in various research and clinical settings to monitor T cell responses, understand immune mechanisms, and develop immunotherapies. By studying these markers, we can gain insights into T cell function and the overall immune response.
In vitro T cell activation method
T cells, a type of white blood cell, play a pivotal role in the immune system by identifying and attacking infected or cancerous cells. Activation of T cells occurs when their T cell receptors (TCRs) recognize and bind to specific antigens presented by antigen-presenting cells (APCs). This interaction triggers a cascade of intracellular signaling events, leading to T cell proliferation, differentiation, and the execution of immune functions such as cytokine production and cytotoxic activity.
In vitro T cell activation is a crucial technique in immunology research, enabling scientists to study T cell responses under controlled laboratory conditions. This method involves stimulating T cells outside the human body to mimic their natural activation process, which is essential for understanding immune responses, developing vaccines, and advancing immunotherapies.
Methods of In Vitro T Cell Activation
Several methods are employed to activate T cells in vitro, with the most common involving the use of antibodies targeting CD3 and CD28 molecules on the T cell surface. These molecules provide the necessary signals for T cell activation and co-stimulation, respectively.
Anti-CD3 and Anti-CD28 Stimulation: This method uses monoclonal antibodies against CD3 and CD28 to mimic the natural activation signals. The antibodies are coated onto culture plates, and T cells are added to these plates. The binding of T cells to the antibodies triggers their activation, leading to proliferation and cytokine production.
Dynabeads: Dynabeads are magnetic beads coated with anti-CD3 and anti-CD28 antibodies. When mixed with T cells, these beads provide a strong and uniform activation signal, promoting T cell expansion. This method is particularly useful for generating large numbers of activated T cells for research and therapeutic purposes.
Chemical Stimulants: Chemicals such as phorbol 12-myristate 13-acetate (PMA) and ionomycin can also be used to activate T cells. PMA activates protein kinase C, while ionomycin increases intracellular calcium levels, both of which are critical for T cell activation. This method is often used in combination with other stimuli to enhance T cell responses.
T cell activation and immunotherapy
T cell activation is a fundamental process in the immune system, playing a critical role in the body’s defense against infections and cancer. Immunotherapy, a revolutionary approach in cancer treatment, leverages this process to enhance the immune system’s ability to target and eliminate cancer cells.
T cells, a type of white blood cell, are essential for adaptive immunity. Their activation begins when T cell receptors (TCRs) recognize and bind to specific antigens presented by antigen-presenting cells (APCs) such as dendritic cells. This interaction is further strengthened by co-stimulatory signals provided by molecules like CD28 on T cells binding to CD80/CD86 on APCs. Once activated, T cells undergo clonal expansion and differentiation into effector T cells, which can directly kill infected or cancerous cells, or helper T cells, which support other immune cells.
Mechanisms of T Cell Activation in Immunotherapy
Immunotherapy aims to harness and enhance the natural power of T cells to fight cancer. There are several strategies to achieve this:
Adoptive Cell Transfer (ACT): This involves extracting T cells from a patient, genetically modifying or expanding them in vitro, and then reintroducing them into the patient. One prominent example is CAR-T cell therapy, where T cells are engineered to express chimeric antigen receptors (CARs) that specifically target cancer cells.
Immune Checkpoint Inhibitors: These drugs block inhibitory pathways that normally keep T cells in check, thereby enhancing their activity against cancer cells. For instance, drugs targeting PD-1/PD-L1 or CTLA-4 pathways have shown significant success in treating various cancers.
T Cell Agonists: These are agents that stimulate T cell activation and proliferation. For example, OX40 agonists can enhance the survival and function of T cells, leading to more robust anti-tumor responses.

