Overview of Monoclonal Antibody Technology
Monoclonal antibody (mAb) technology has Developed by Georges Köhler and César Milstein in 1975, this technology involves producing antibodies from a single clone of B-lymphocytes, ensuring high specificity. Unlike polyclonal antibodies, which recognize multiple epitopes, monoclonal antibodies target a single epitope, making them highly precise tools in medical applications.
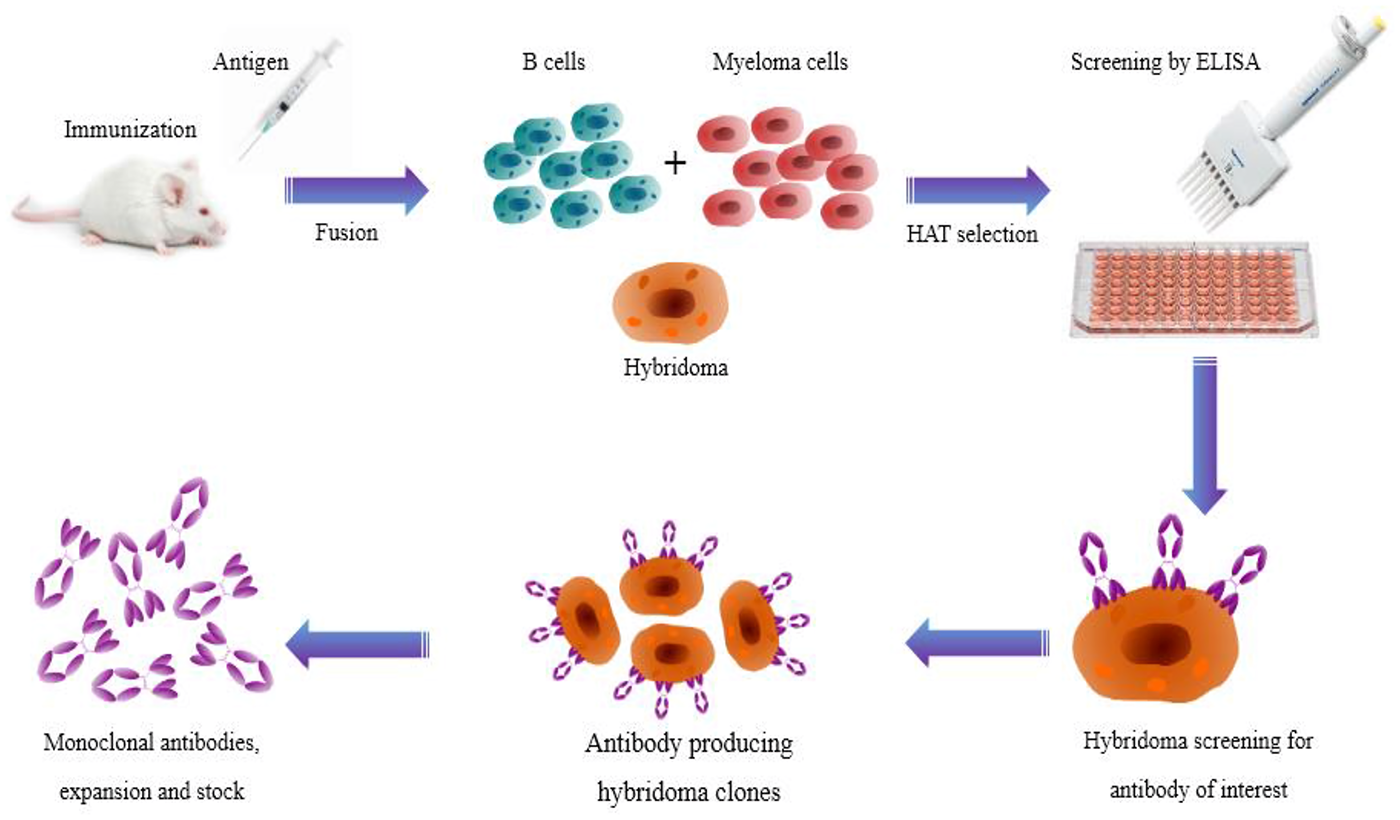
The process of producing a monoclonal antibody begins by immunizing an animal (usually a mouse) with the target antigen. B lymphocytes from the animal's spleen are then fused with myeloma cells to produce hybridomas. These hybridomas are screened and cloned to produce the desired antibody.
Importance of Monoclonal Antibody Technology in Scientific Research and Medicine
Monoclonal antibody (Mab) technology has become an important part of scientific research and medicine due to its high specificity and versatility.
In scientific research, monoclonal antibodies are indispensable tools for identifying and isolating specific proteins, studying cellular processes, and developing diagnostic tests. Their ability to bind to a single epitope on an antigen makes them highly specific, reducing the likelihood of cross-reactivity and false results.
In medicine, monoclonal antibodies have revolutionized the treatment of various diseases, including cancer, autoimmune disorders, and infectious diseases. For instance, monoclonal antibodies are used in targeted cancer therapies to bind specific antigens on cancer cells, leading to their destruction. According to recent data, monoclonal antibodies represent the fastest-growing category of therapeutics entering clinical studies worldwide. They offer several advantages over traditional drugs, including increased specificity, longer half-life, and reduced adverse effects.
Moreover, monoclonal antibodies have been pivotal in the development of vaccines and treatments for emerging infectious diseases, such as COVID-19. Their ability to neutralize pathogens and modulate the immune response has made them valuable assets in combating global health threats.
Main Types of Monoclonal Antibody Technology
Monoclonal antibody technology includes murine, chimeric, humanized, and human antibodies. Murine antibodies are 100% mouse-derived, while chimeric antibodies combine mouse and human elements. Humanized antibodies are mostly human with minimal mouse components, and human antibodies are fully human. These types enhance specificity and reduce adverse reactions.
Hybridoma Technology
Hybridoma technology, developed by Köhler and Milstein in 1975, is a pivotal method for producing monoclonal antibodies (mAbs). This technique involves fusing antibody-producing B lymphocytes with myeloma cells to create hybrid cells, known as hybridomas. These hybridomas can be cultured to produce large quantities of specific antibodies.
Advantages
High Specificity and Sensitivity: Hybridoma technology produces antibodies that are highly specific and sensitive to the target antigen.
Limitless Production: Once hybridoma cells are stabilized, they can produce homogenized antibodies indefinitely.
Therapeutic Applications: Over 80 monoclonal antibodies generated from hybridomas have been approved for therapeutic use worldwide.
Cost-Effective: Despite the initial setup costs, the long-term production of antibodies is cost-effective.
Disadvantages
Long Production Time: The process can take 6-8 months to produce a reasonable amount of monoclonal antibodies.
Resource-Intensive: The workflow is expensive and requires significant resources.
Contamination Risks: There is a risk of virus contamination and disease transmission from the murine origin of the antibodies.
Humanization Requirement: Antibodies of murine origin need further humanization for therapeutic purposes, adding extra costs.
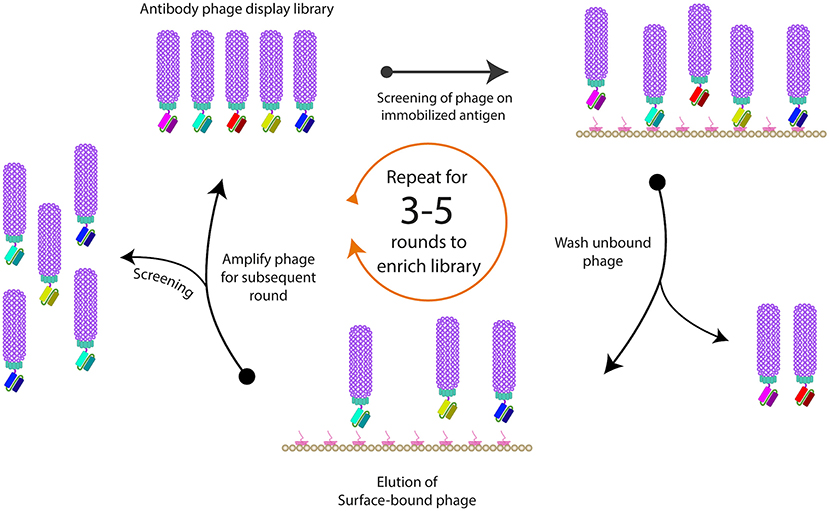
Phage Display Technology
Phage display technology, introduced by George P. Smith in 1985, is a powerful method for studying protein interactions and developing therapeutic antibodies. This technique involves displaying peptides or proteins on the surface of bacteriophages, allowing for the identification of high-affinity ligands through iterative selection processes.
Advantages
Large Library Size: Phage display can generate libraries with up to 10^12 variants, providing a vast pool for selecting high-affinity binders.
Versatility: It is used in various fields, including drug discovery, diagnostics, and vaccine development.
Cost-Effective: Compared to other display technologies, phage display is relatively inexpensive and easy to implement.
Rapid Screening: The technology allows for quick identification and optimization of binding molecules.
Disadvantages
Lower Affinity: Binders selected from naïve libraries may exhibit lower affinity compared to those from immunized libraries.
Complexity: The process can be technically challenging, requiring expertise in molecular biology and bioinformatics.
Potential Bias: There is a risk of selection bias, where certain sequences may be overrepresented.
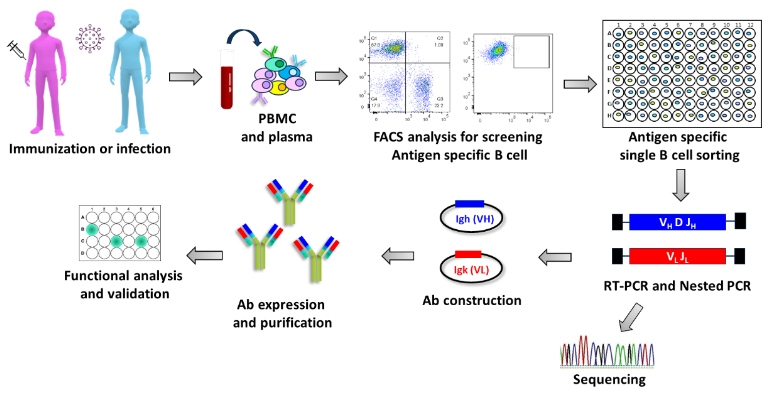
Single B Cell Technology
Single B cell technology is a cutting-edge method for discovering monoclonal antibodies (mAbs). This technique involves isolating individual B cells that produce specific antibodies, allowing for the direct amplification of genes encoding the variable regions of these antibodies.
Advantages
High Throughput: Single B cell platforms can screen thousands of individual clones in a short period, making it possible to isolate rare binders efficiently.
Precision: This technology captures the entire B cell compartment, ensuring that even rare and potentially valuable antibodies are identified.
Speed: The process is faster compared to traditional methods like hybridoma technology, reducing the time required for antibody discovery.
Humanization: Antibodies derived from human B cells are more likely to be compatible with human therapeutic use, reducing the need for further humanization.
Disadvantages
High Costs: The R&D costs associated with single B cell screening technology are higher due to the requirement for specialized equipment and expertise.
Technical Complexity: The process can be technically challenging, requiring advanced knowledge in molecular biology and bioinformatics.
Limited Epitope Diversity: There may be limitations in the diversity of epitopes that can be targeted, depending on the B cell repertoire.
Short-lived B Cells: B cells derived from different hosts are notably short-lived in ex vivo culture conditions, making them difficult to interrogate.
Transgenic Animal Models - Advantages and disadvantages
Transgenic animal models have revolutionized biomedical research by allowing scientists to study gene functions and disease mechanisms in vivo. These models are created by introducing foreign genes into the genome of an animal, resulting in the expression of new traits.
Advantages
Disease Research: Transgenic animals are invaluable for studying human diseases, including cancer, diabetes, and neurodegenerative disorders. For example, transgenic mice have been used to model Alzheimer’s disease, providing insights into its pathogenesis.
Drug Development: These models facilitate the development and testing of new drugs, leading to more effective treatments. Transgenic animals can be engineered to express human proteins, making them ideal for preclinical testing.
Agricultural Benefits: Transgenic livestock can be engineered for improved traits such as disease resistance, faster growth, and enhanced milk production. This can lead to increased agricultural productivity and food security.
Disadvantages
Ethical Concerns: The creation and use of transgenic animals raise ethical issues related to animal welfare and the potential for unintended consequences. There is ongoing debate about the moral implications of genetic manipulation.
High Costs: The process of creating and maintaining transgenic animals is expensive and resource-intensive. This includes costs associated with specialized equipment, facilities, and personnel.
Technical Challenges: The generation of transgenic animals can be technically complex, with potential issues such as low efficiency of gene integration and variable expression levels. Additionally, there may be high mortality rates and other deleterious effects on the animals.
Guidelines for Choosing Monoclonal Antibody Technology
When selecting a monoclonal antibody, consider its specificity, affinity, and production method. Data shows that antibodies with high affinity and specificity yield better therapeutic outcomes. Additionally, ensure the production process adheres to regulatory guidelines to maintain safety and efficacy.
Define Application Purpose
The first step in selecting monoclonal antibody technology is to clearly define the purpose of the application. Are you developing a therapeutic agent, a diagnostic tool, or a research reagent? For therapeutic applications, the mAb must be highly specific and effective against the target disease. For diagnostic purposes, the mAb should provide accurate and reliable results. Understanding the application purpose helps in setting clear objectives and guides the selection process.
Consider Specificity and Affinity Requirements
Specificity and affinity are critical parameters in the development of monoclonal antibodies. Specificity refers to the ability of the antibody to bind exclusively to a particular antigen, while affinity measures the strength of this binding. High specificity and affinity are essential for the effectiveness of mAbs in both therapeutic and diagnostic applications. According to a study published in Nature Reviews Drug Discovery, mAbs with high specificity and affinity have a higher success rate in clinical trials, with a success rate of approximately 20% compared to 11% for small molecule drugs.
Evaluate Production Scalability
Production scalability is another important consideration. This involves assessing whether the monoclonal antibody can be produced on a large scale without compromising quality or performance. Factors to consider include the availability of raw materials, manufacturing processes, and cost-effectiveness. Data from the Biotechnology Innovation Organization (BIO) indicates that only 30% of biopharmaceuticals successfully scale from pilot to full-scale production. Therefore, it is crucial to evaluate the scalability of the mAb production process early in the development phase.
Humanization and Immunogenicity
Humanization and immunogenicity are critical factors in the development of therapeutic monoclonal antibodies. Humanization refers to the process of modifying non-human antibodies to make them more similar to human antibodies, thereby reducing the risk of immune reactions. Immunogenicity is the ability of a substance to provoke an immune response. High immunogenicity can lead to adverse effects and reduce the efficacy of the treatment. According to a study published in the Journal of Immunology, humanized antibodies have a significantly lower immunogenicity compared to their non-human counterparts, making them more suitable for therapeutic use.
Budget and Time Constraints
Finally, budget and time constraints are key factors that can impact the development and implementation of monoclonal antibody technology. Developing a new mAb, especially for therapeutic use, can be expensive and time-consuming. It is important to have a clear budget and timeline to ensure that the project stays on track. Data from a report by the Tufts Center for the Study of Drug Development shows that the average cost of developing a new drug is approximately $2.6 billion, and the process can take up to 10 years. These constraints must be carefully managed to ensure the successful completion of the project.
Monoclonal antibody (mAb) technology has transformed biotechnology and medicine by providing targeted therapies for a wide range of diseases. These antibodies are designed to specifically bind to antigens with high specificity and affinity, thereby enhancing their effectiveness. Humanization of mAbs reduces immunogenicity, making them safer for therapeutic use. However, developing mAbs involves huge budgetary and time constraints, with an average cost of $2.6 billion and a development time of up to 10 years. Despite these challenges, mAb technology remains a promising avenue for innovative therapeutics and diagnostics, and its success rate in clinical applications continues to increase.