The His tag, short for polyhistidine tag, is a sequence of six to ten histidine residues added to proteins to facilitate purification and detection. This tag binds strongly to metal ions like nickel or cobalt, allowing tagged proteins to be isolated using immobilized metal affinity chromatography (IMAC). His tags are widely used in biochemical research due to their simplicity, efficiency, and minimal impact on protein function. They enable researchers to purify proteins from complex mixtures, study protein-protein interactions, and perform structural analyses.
Understanding the Role of His Tag in Biochemical Research
Protein Purification
The His tag, also known as the polyhistidine tag, is widely used for protein purification due to several key advantages:
Small Size: The His tag is relatively small (usually 6-10 histidine residues), which minimizes the likelihood of interfering with the protein’s native structure and function.
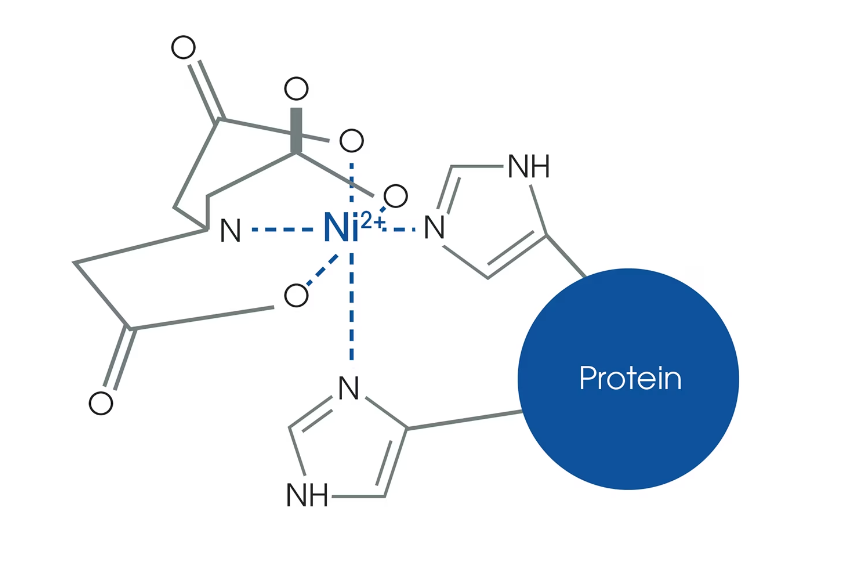
High Affinity and Specificity: His tags bind strongly and specifically to divalent metal ions like nickel (Ni²⁺) and cobalt (Co²⁺), allowing for efficient purification using immobilized metal affinity chromatography (IMAC).
Versatility: His tags can be added to either the N- or C-terminus of the protein, and they work well under both native and denaturing conditions.
Ease of Use: The purification process using His tags is straightforward and can be performed under a variety of conditions, making it suitable for a wide range of proteins.
Commercial Availability: There are many commercially available reagents and kits designed specifically for His-tagged protein purification, which simplifies the process.
Detection
The His tag binds strongly to metal ions and is compatible with a variety of detection methods:
Metal Ion Binding: The His tag binds specifically to divalent metal ions like nickel (Ni²⁺) and cobalt (Co²⁺), which allows for the use of immobilized metal affinity chromatography (IMAC) to isolate and detect His-tagged proteins.
Antibody Detection: Anti-His tag antibodies are commercially available and can be used in various assay methods, such as Western blotting, ELISA, and immunoprecipitation, to detect His-tagged proteins without the need for a protein-specific antibody.
Versatility: The His tag can be detected under both native and denaturing conditions, making it a versatile tool for various experimental setups.
Ease of Use: The detection process is straightforward and can be performed with readily available reagents and kits, simplifying the workflow for researchers.
Protein-Protein Interaction Studies
The His tag, or polyhistidine tag, is a short sequence of histidine residues added to proteins to facilitate their purification and detection. In protein-protein interaction studies, the His tag plays several important roles:
Purification: The His tag allows for the efficient purification of recombinant proteins using immobilized metal affinity chromatography (IMAC). This technique exploits the affinity of histidine residues for metal ions, such as nickel or cobalt, enabling the selective isolation of His-tagged proteins from complex mixtures.
Detection: His-tagged proteins can be easily detected using anti-His antibodies in techniques like Western blotting, ELISA, or immunoprecipitation. This simplifies the identification and analysis of proteins involved in interactions.
Immobilization: His tags can facilitate the immobilization of proteins on metal-coated surfaces, which is useful for studying protein-protein interactions in various assays, such as surface plasmon resonance (SPR) or enzyme-linked immunosorbent assays (ELISAs).
Solubility and Stability: The His tag can improve the solubility and stability of recombinant proteins, which is beneficial for maintaining the functional integrity of proteins during interaction studies.
Structural Analysis
The His tag, or polyhistidine tag, is widely used in protein structural analysis for several key reasons:
Purification: The His tag facilitates the purification of recombinant proteins using immobilized metal affinity chromatography (IMAC). This ensures that the protein sample is highly pure, which is crucial for accurate structural analysis.
Crystallization: High purity of His-tagged proteins aids in the crystallization process, which is essential for X-ray crystallography. The His tag can also be used to immobilize proteins on surfaces, aiding in the formation of crystals.
Stability: The His tag can enhance the solubility and stability of proteins, which is beneficial for maintaining their native conformation during structural studies.
Detection: His-tagged proteins can be easily detected and quantified using anti-His antibodies, simplifying the analysis of protein samples.
His-Tag Applications
When extremely high purity of the protein is not required, His-Tag fusion protein purification can be used as the only purification step. When the highest purity is required, this technique can be used as the first (capture) step in a multi-step purification process. It is also suitable for purifying dual-tagged proteins with a histidine tag on one end and a different tag on the other end.
The His-Tag does not usually need to be removed. In some cases, when it interferes with the function of the target protein or the target protein needs to be in a native state, the His-Tag can be removed on the resin of the second purification of the target protein using a recombinant protease.
How much histidine should be added for protein expression and production?
The number of histidine residues in the poly-His tag affects the binding of the tagged protein to the affinity resin and the elution of the protein from the affinity column. Typically, one hexa-His tag is sufficient for affinity purification and antibody detection. However, sometimes 7-10 histidines are used to increase the binding of the His-Tagged protein to the affinity column, possibly because one or more of the histidine residues are not exposed to the resin.
His-Tag Enzyme Cleavage Methods
In some applications, the His-Tag needs to be removed, such as protein crystallization. To cleave the tag, a protease cleavage site needs to be designed between the tag and the protein. The EK cleavage site after the His tag (poly-his-EK site-protein structure) allows the His tag and cleavage site to be completely removed, leaving no additional amino acids after specific cleavage of the His tag.

