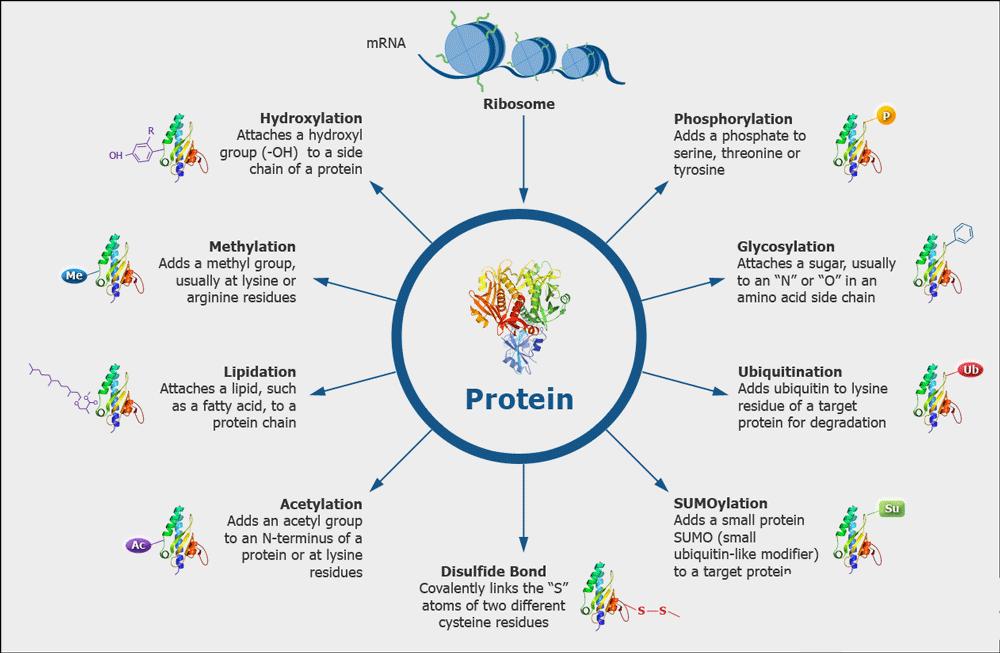
Proteins are essential macromolecules that play a vital role in nearly all biological processes. They are the building blocks of life and are responsible for the structure, function, and regulation of body tissues and organs. Proteins act as enzymes, hormones, and antibodies, facilitating biochemical reactions, signaling pathways, and immune responses. Their diverse functions make them indispensable for the growth, repair, and maintenance of cells, as well as for overall health and development.
Protein characterization refers to the comprehensive analysis and identification of a protein's structure, function, and interactions. This process is important because it provides insight into the role of proteins in biological systems, aiding in the understanding of disease mechanisms and the development of therapeutic interventions. By characterizing proteins, scientists can identify potential drug targets, design effective treatments, and advance biotechnology applications.

Basic Methods of Protein Characterization
Protein characterization is essential for understanding the structure, function, and interactions of proteins. Several techniques are commonly used to analyze proteins, each with its unique advantages and applications. Here, we discuss four fundamental methods: Mass Spectrometry, X-ray Crystallography, Nuclear Magnetic Resonance (NMR) Spectroscopy, and Circular Dichroism (CD) Spectroscopy.
Mass Spectrometry
Mass spectrometry (MS) is a powerful analytical technique used to measure the mass-to-charge ratio of ions. It is widely used for protein identification and quantification. MS can analyze complex protein mixtures, providing information on molecular weight, post-translational modifications, and protein-protein interactions. The sensitivity and accuracy of MS make it an indispensable tool in proteomics. For example, tandem mass spectrometry (MS/MS) can sequence peptides with high precision, aiding in the identification of proteins in complex biological samples.
X-ray Crystallography
X-ray crystallography is a technique used to determine the atomic structure of proteins. By crystallizing a protein and exposing it to X-ray radiation, researchers can obtain diffraction patterns that reveal the protein’s three-dimensional structure. This method has been instrumental in elucidating the structures of many important proteins, including enzymes and membrane proteins. The high-resolution data obtained from X-ray crystallography provide detailed insights into protein function and interactions. For instance, the structure of the ribosome was determined using this technique, advancing our understanding of protein synthesis.
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy is a technique that exploits the magnetic properties of atomic nuclei to determine the structure and dynamics of proteins in solution. Unlike X-ray crystallography, NMR does not require protein crystallization, making it suitable for studying proteins in their native state. NMR provides information on protein folding, conformational changes, and interactions with other molecules. It is particularly useful for studying small to medium-sized proteins. For example, NMR has been used to investigate the structure of intrinsically disordered proteins, which play crucial roles in cellular signaling.
Circular Dichroism (CD) Spectroscopy
Circular dichroism (CD) spectroscopy measures the differential absorption of left- and right-handed circularly polarized light by chiral molecules, such as proteins. CD spectroscopy is used to analyze protein secondary structures, such as alpha-helices and beta-sheets. It provides information on protein folding and stability, as well as conformational changes induced by ligand binding or environmental conditions. CD spectroscopy is a rapid and non-destructive technique, making it ideal for monitoring protein folding in real-time. For instance, CD has been used to study the folding kinetics of lysozyme, a model protein.
Application of Protein Characterization
Protein characterization is a crucial aspect of modern biological sciences, providing insights into the structure, function, and interactions of proteins. This knowledge is pivotal in various fields, including drug development, disease diagnosis, and biotechnology research.
Drug Development
In drug development, protein characterization plays a vital role in identifying and validating drug targets. By understanding the structure and function of proteins involved in disease pathways, researchers can design drugs that specifically interact with these targets. Techniques such as X-ray crystallography, mass spectrometry, and nuclear magnetic resonance (NMR) spectroscopy are commonly used to determine the three-dimensional structures of proteins. This structural information aids in the rational design of small molecules or biologics that can modulate protein activity, leading to the development of effective therapeutics. Additionally, protein characterization helps in assessing the stability, purity, and activity of drug candidates, ensuring their safety and efficacy.
Disease Diagnosis
Protein characterization is also essential in disease diagnosis. Biomarkers, which are proteins associated with specific diseases, can be identified and quantified using various techniques. For instance, enzyme-linked immunosorbent assays (ELISA) and mass spectrometry are employed to detect and measure the levels of disease-specific proteins in biological samples. These biomarkers can provide valuable information about the presence and progression of diseases, enabling early diagnosis and personalized treatment plans. Furthermore, protein characterization can reveal post-translational modifications, such as phosphorylation or glycosylation, which are often altered in disease states. Understanding these modifications can lead to the development of diagnostic tests and therapeutic strategies.
Biotechnology Research
In biotechnology research, protein characterization is fundamental for the development of novel biotechnological applications. For example, the production of recombinant proteins, such as enzymes, antibodies, and hormones, relies on detailed knowledge of protein structure and function. Techniques like circular dichroism (CD) spectroscopy and differential scanning calorimetry (DSC) are used to study protein folding, stability, and interactions. This information is crucial for optimizing protein expression, purification, and formulation processes. Additionally, protein characterization aids in the engineering of proteins with enhanced properties, such as increased stability or altered specificity, which can be utilized in various industrial and therapeutic applications.
Challenges in Protein Characterization
Protein characterization is a critical process in biological research, but it comes with several challenges that can impact the accuracy and reliability of results. Three primary challenges are sample purity, data analysis complexity, and technical limitations.
Sample Purity
Ensuring the purity of protein samples is essential for accurate characterization. Contaminants such as other proteins, nucleic acids, or small molecules can interfere with analytical techniques, leading to misleading results. Achieving high purity often requires multiple purification steps, which can be time-consuming and may result in sample loss or degradation.
Data Analysis Complexity
The complexity of data analysis is another significant challenge in protein characterization. Techniques such as mass spectrometry and nuclear magnetic resonance (NMR) spectroscopy generate vast amounts of data that require sophisticated software and expertise to interpret. Identifying and quantifying proteins, as well as understanding their post-translational modifications and interactions, demands advanced computational tools and bioinformatics approaches.
Technical Limitations
Technical limitations of current analytical methods also pose challenges. For instance, some techniques may lack the sensitivity to detect low-abundance proteins or the resolution to distinguish between closely related protein isoforms. Additionally, the structural complexity of proteins, including their dynamic nature and conformational flexibility, can make it difficult to obtain detailed structural information.
Future Directions for Protein Characterization
The potential for protein characterization is enormous, aided by advances in technology and methods. Improvements in the sensitivity and resolution of analytical techniques will allow us to detect and analyze low-abundance proteins and subtle structural changes. The integration of artificial intelligence and machine learning will simplify data analysis, providing a deeper understanding of protein function and interactions. In addition, innovations in single-molecule technology will allow real-time monitoring of protein dynamics and conformational changes. These developments will greatly improve our understanding of proteins, leading to breakthroughs in drug discovery, disease diagnostics, and biotechnology applications.

